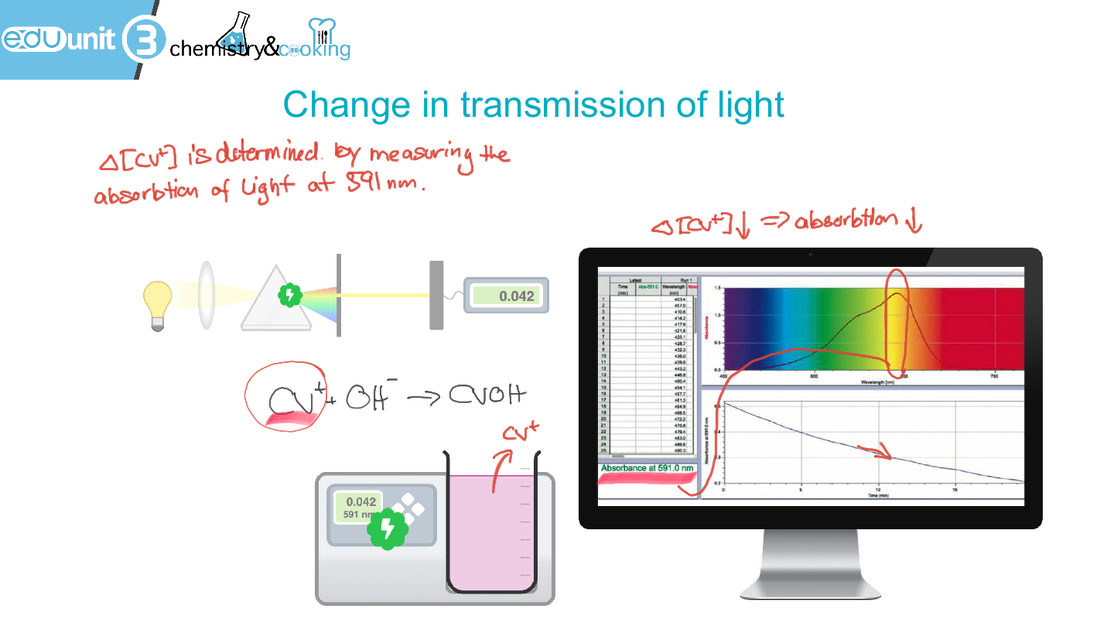
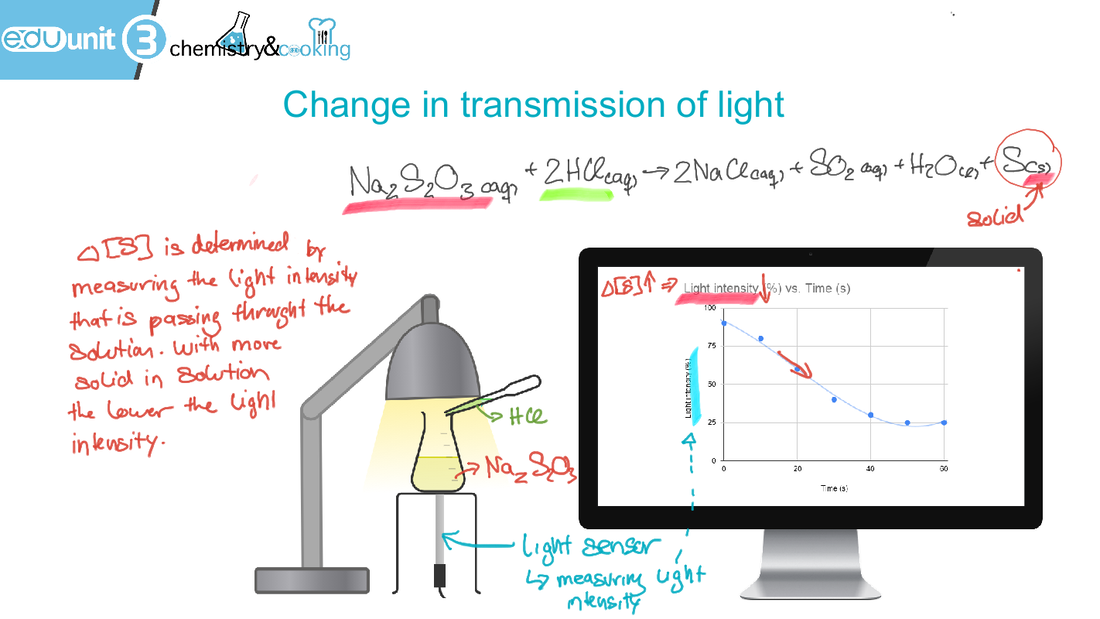
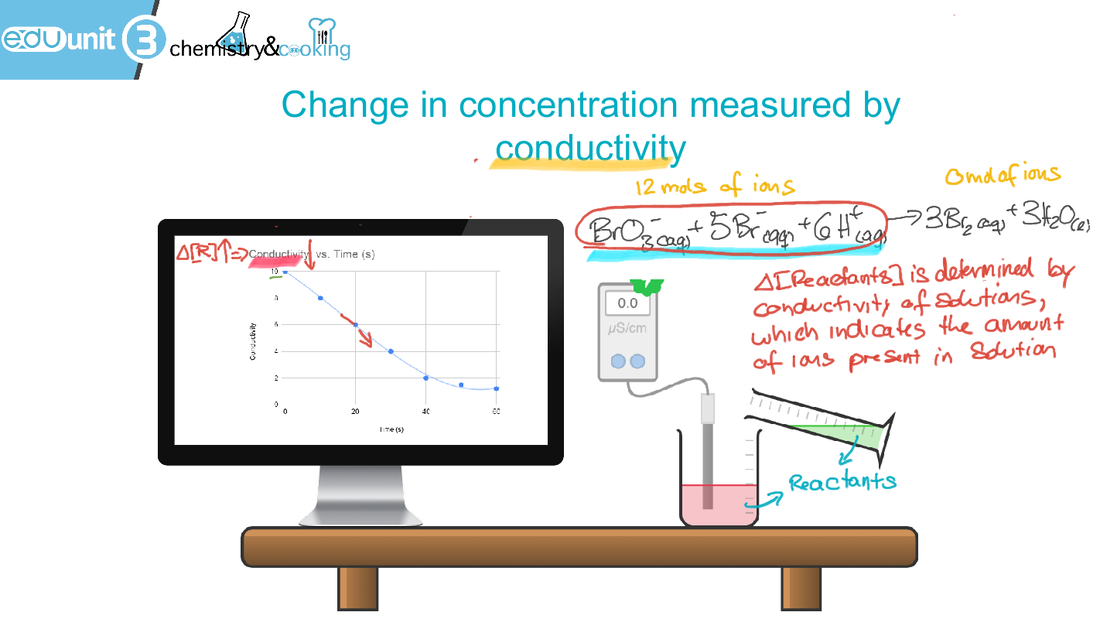
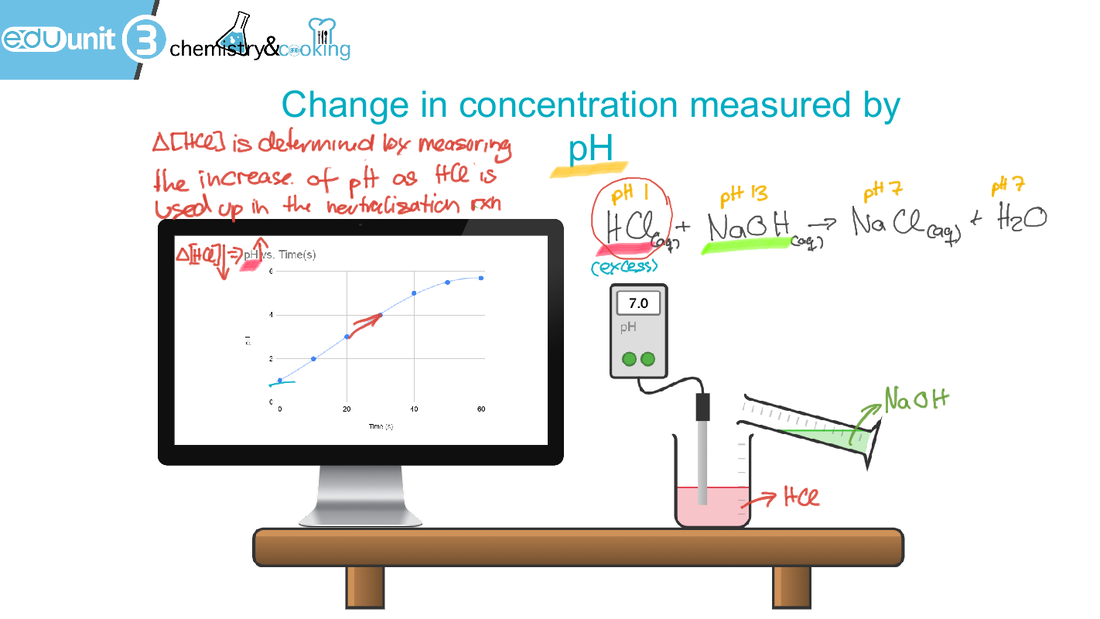
6.1 Collision theory and rates of reaction
|
|
Additional resources
Please note that this is student work and is included here as it was presented
Task:
Watch the video and complete the tasks within the video.
Watch the video and complete the tasks within the video.
|
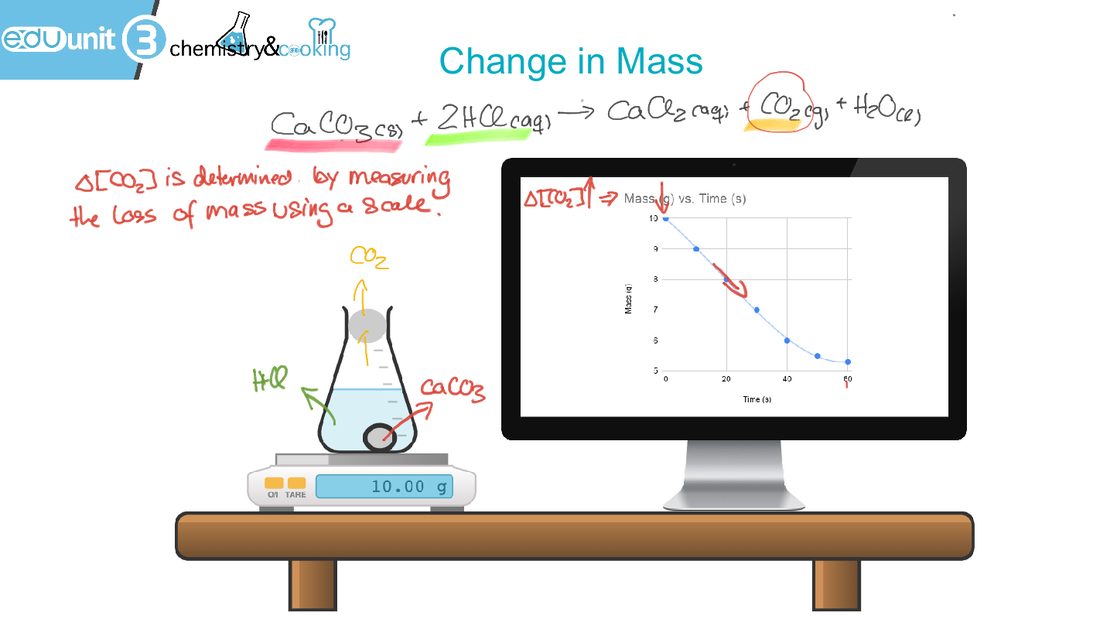
The reaction of Baking soda and vinegar is another very common reaction in the kitchen.
Task:
|
|
The Maillard reaction is occurring everyday in the kitchen if you cook a steak, for example, of back cookies. Let's investigate how we can control the rate of this reaction.
Video of the Maillard reaction - Bakery
Task:
- Check out the poster and watch the video above
- Plan your own practical which investigates how to speed up the maillard reaction (use IBDP Exploration criterion) - consider the following 3 factors:
- Change of Heat
- Change of Concentration
- Change of pH
- Conduct your cooking lab (it is important to note that you are only able to measure the rate qualitative by observing the colour of the bakery). Be sure to video record your practical.
- Produce a table of results
- Evaluate your method (use IBDP Evaluation) criterion
- Complete your video of your student lab, which should contain the following:
- Introduction to your experiment (explain what you are going to do and state your research question, Variables and equipment/ingredients)
- Video footage of the actual practical with your step by step method as caption
- Conclusion to your experiment (show your table to results, as well as pictures/video of your results and explain in short strength and weaknesses of the practical as well as improvements)
- Use Flipgrid to upload your video (max 10min)
- Give peer feedback
|
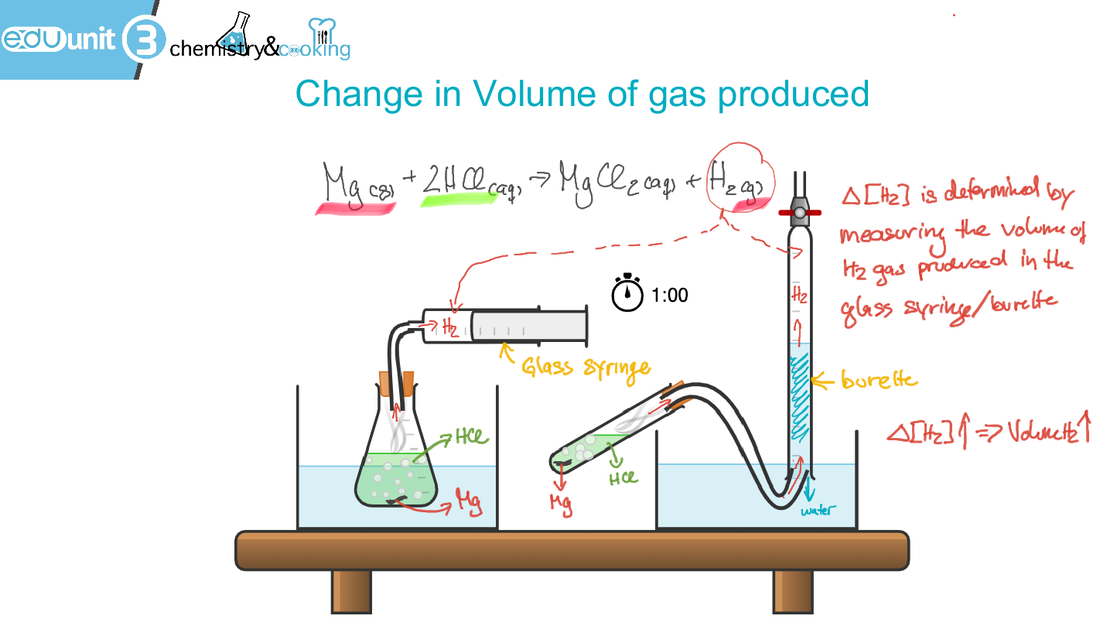
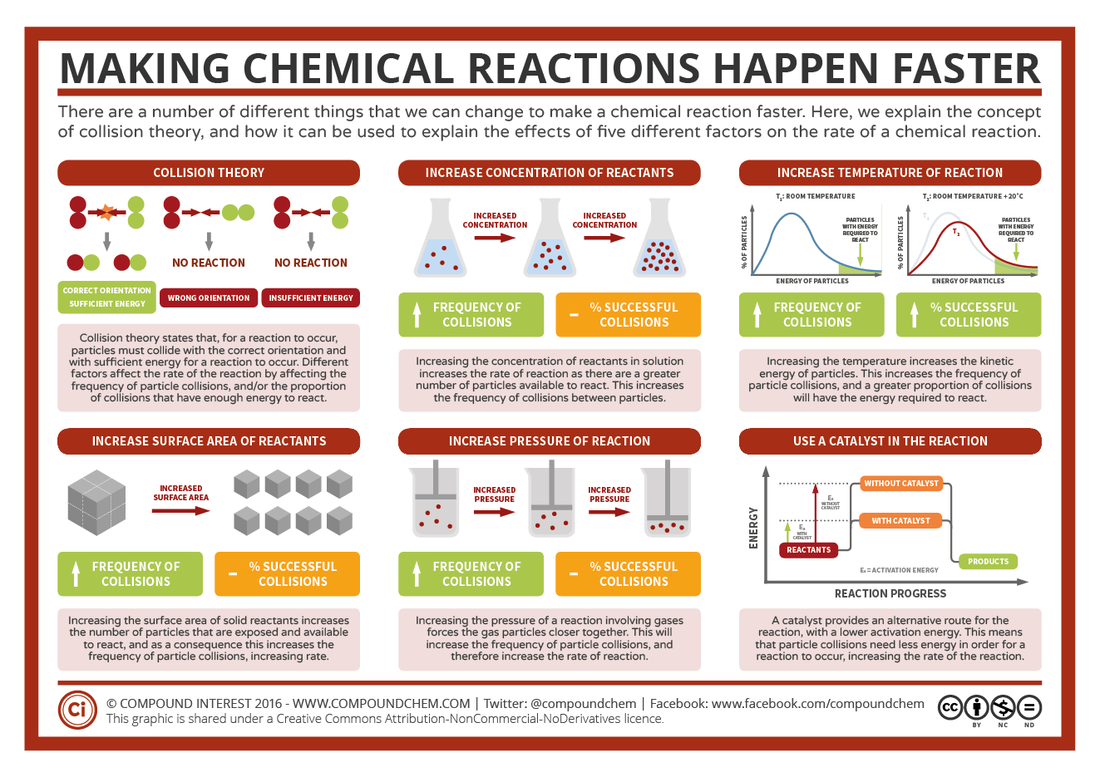
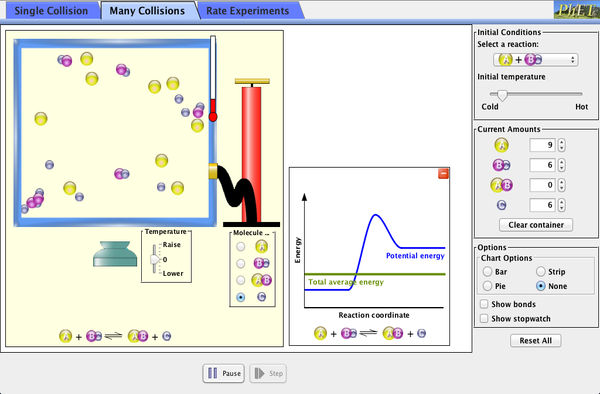
(There are a number of factors that can effect the rate of reaction:
Task:
|
Overview of factors
Focus on Collision theory
Focus on Maxwell Boltzmann distribution curve
|