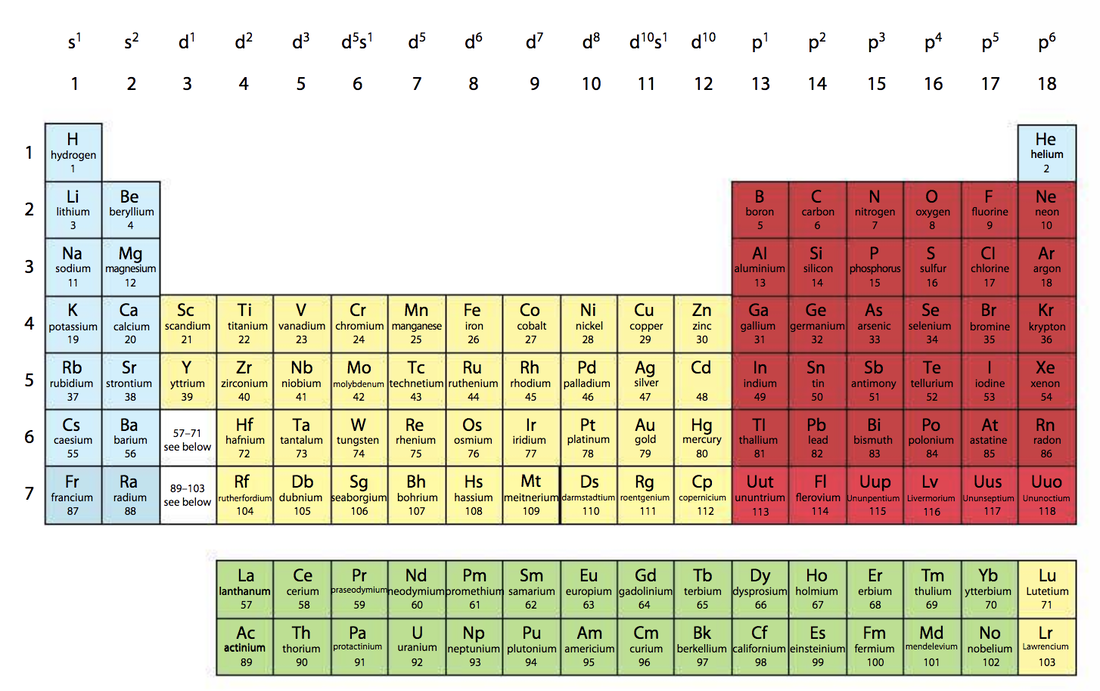
Understandings● The Periodic Table is arranged into four blocks associated with the four sub-levels: s, p, d, and f.
● The Periodic Table consists of groups (vertical columns) and periods (horizontal rows). Guidance The group numbering scheme from group 1 to group 18, as recommended by IUPAC, should beused. ● The period number (n) is the outer energy level that is occupied by electrons. ● The number of the principal energy level and the number of the valence electrons in an atom can be deduced from its position on the Periodic Table. ● The Periodic Table shows the positions of metals, non-metals and metalloids. Guidance The terms alkali metals, halogens, noble gases, transition metals, lanthanoides and actinoides should be known. |
Applications and skills● Deduction of the electron configuration of an atom from the element’s position on the Periodic Table, and vice versa.
|
3.1 Periodic table
|
|
Information form the periodic tableThese videos explains what information is in the periodic table besides the name of elements, symbols, mass number and proton number. Periods, groups and blocks all give important information about a certain element.
|