|
● Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron
transfer, or change in oxidation number. ● An oxidizing agent is reduced and a reducing agent is oxidized. Guidance Oxidation states should be represented with the sign given before the number, e.g. +2 not 2+. ● Variable oxidation numbers exist for transition metals and for most main-group non-metals. Guidance The oxidation state of hydrogen in metal hydrides (–1) and oxygen in peroxides (–1) should be covered. ● The activity series ranks metals according to the ease with which they undergo oxidation. Guidance A simple activity series is given in the IB data booklet in section 25. ● The Winkler method can be used to measure biochemical oxygen demand (BOD), used as a measure of the degree of pollution in a water sample. |
● Deduction of the oxidation state of an atom in an ion or a compound.
● Deduction of the name of a transition metal compound from a given formula, applying oxidation numbers represented by Roman numerals. Guidance Oxidation number and oxidation state are often used interchangeably, though IUPAC does formally distinguish between the two terms. Oxidation numbers are represented by Roman numerals according to IUPAC. ● Identifi cation of the species oxidized and reduced and the oxidizing and reducing agents, in redox reactions. ● Deduction of redox reactions using half-equations in acidic or neutral solutions. ● Deduction of the feasibility of a redox reaction from the activity series or reaction data. ● Solution of a range of redox titration problems. ● Application of the Winkler method to calculate BOD. |
Task:
- Watch the video and take notes
Task:
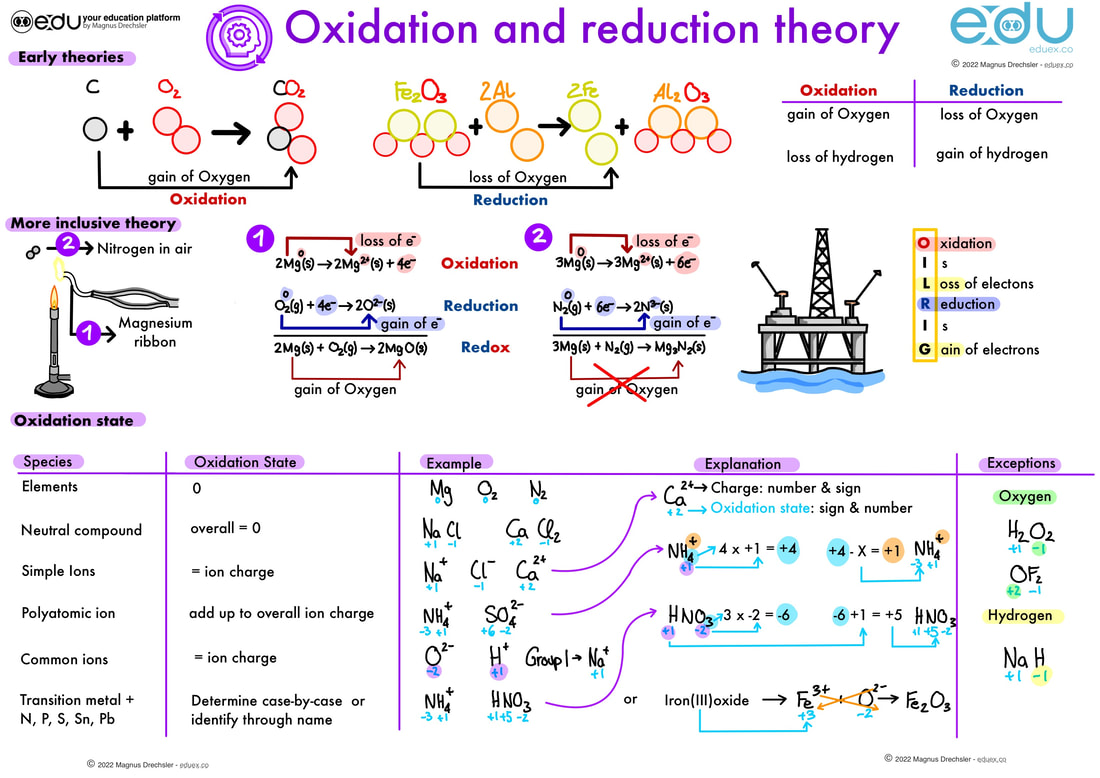
Look at the image above and complete the following tasks for each section
Early theories:
More inclusive theory:
Oxidation state:
Look at the image above and complete the following tasks for each section
Early theories:
- Describe how oxidation and reduction was defined early on
More inclusive theory:
- Describe how oxidation and reduction is defined in this theory
- Explain how this theory is more inclusive than the early theories
Oxidation state:
- Use the rules above to determine the oxidation state in the following species:
- H2SO4
- SO3 2-
- [Co(NH3)6]3+
- Chromium(III)oxide
|
What is oxidative stress and antioxidants?
Task:
|
|
|
Preventing oxidative stress through our diet
Task:
|
|
Task:
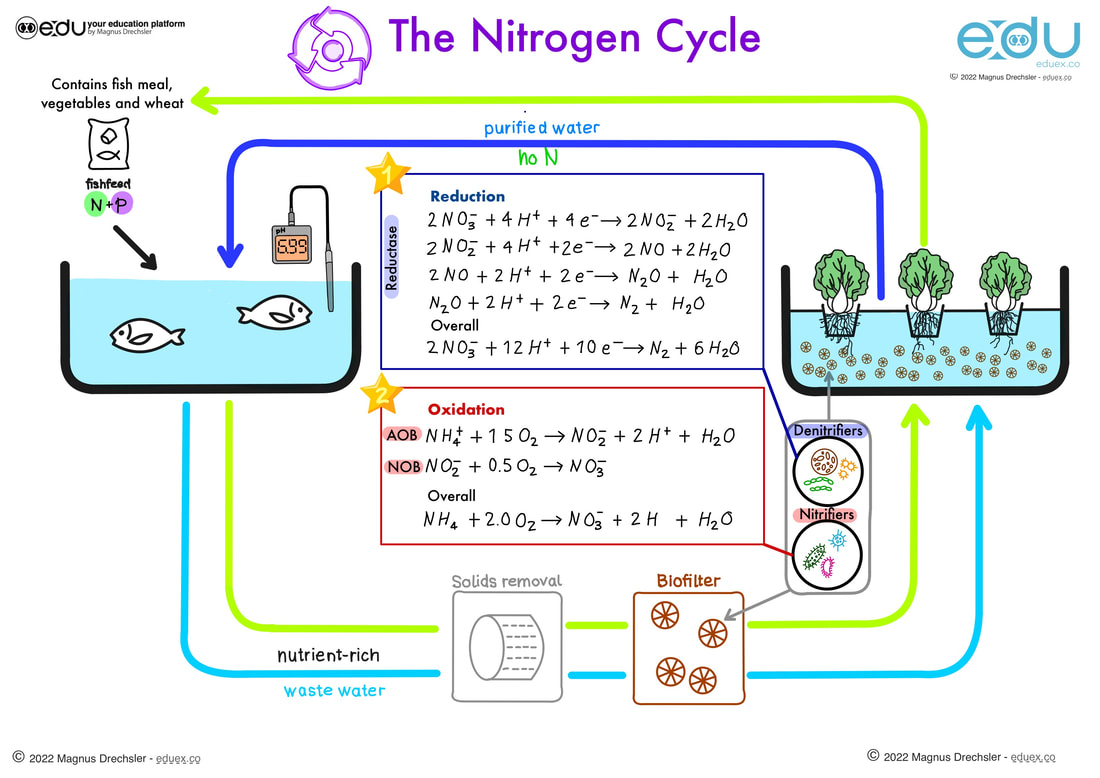
Look at the image and complete the following task (indicated by a star in the image)
STAR 1:
Add information about the nitrogen cycle to your Aquaponics presentation
Look at the image and complete the following task (indicated by a star in the image)
STAR 1:
- Identify the oxidation states of all the species in the reduction equations
- Explain how one knows how many electrons were gained in this reduction and which element gained the electrons
- Identify the oxidation states of all the species in the oxidation equations
- Determine the amount of electrons lost in the oxidation reactions and add them to the correct side of the balanced equation
Add information about the nitrogen cycle to your Aquaponics presentation
|
This video is introducing the basics of oxidation and reduction as well as explains what oxidation numbers are and how they are useful in redox reactions.
Task:
|
|
Redox titration |
|
Method
- Fill a burette with the potassium iodate solution, KIO3(aq), and measure 5.00 cm3 into a conical flask.
- Into the same conical flask pour about 10 cm3 of 1.00 mol dm–3 potassium iodide solution, KI, and add about 10 cm-3 of 2.0 mol dm–3 HCl.
- Allow the reaction to go to completion and record all changes.
- Fill a burette with the 0.10 mol dm–3 sodium thiosulfate solution, Na2S2O3, and titrate it against the iodine in the conical flask.
- When the colour of the iodine has almost gone, add 1–2 cm3 of the starch suspension, and continue the addition of Na2S2O3 drop-wise until the blue colour disappears. Record the volume added.
- Repeat the experiment until consistent titration results are obtained.
Task:
- Get in groups and choose a reaction from the following
- Analysis of iron with manganate
- Iodine-thiosulfate reaction
- Winkler method
- Research your reaction
- Prepare to present your findings