|
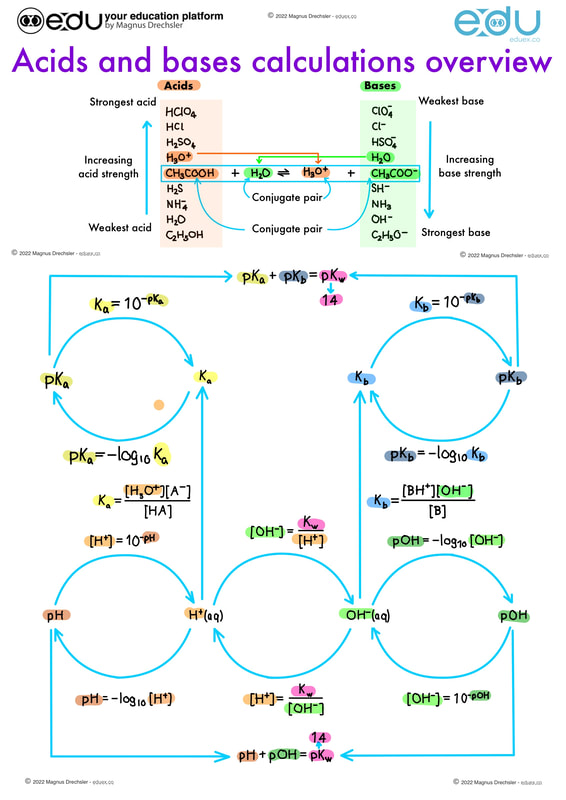
● The expression for the dissociation constant of a weak acid (Ka) and a weak base (Kb).
● For a conjugate acid–base pair, Ka Å~ Kb = Kw. Guidance ● The value Kw depends on the temperature. ● Only examples involving the transfer of one proton will be assessed. ● Calculations of pH at temperatures other than 298 K can be assessed. ● The relationship between Ka and pKa is pKa = –logKa and between Kb and pKb is pKb = –logKb. |
● Solution of problems involving [H+(aq)], [OH–(aq)], pH, pOH, Ka, pKa, Kb, and pKb.
Guidance ● Students should state when approximations are used in equilibrium calculations. ● The use of quadratic equations will not be assessed. ● Discussion of the relative strengths of acids and bases using values of Ka, pKa, Kb, and pKb. Guidance The calculation of pH in buffer solutions will only be assessed in options B.7 and D.4. |
|
18.2 What are the dissociation constant for weak acids and bases
18.2 What is the relationship between Ka and pKa is pKa = –log Ka and between Kb and pKb is pKb = –log Kb? |
18.2 How can [H + (aq)], [OH – (aq)], pH, pOH, Ka , pKa , Kb , and pKb be identified in different acid base problems?
18.2 How can you calculate the pH in a buffer solution? |
18.2 To what extent are buffer solution used in industry?
|
Calculations involving Acids and Bases
|
Explanation how pH and Kw are linked
Follow the exercise that explains how you can determine Kw
Explanation on Acid Strength
Follow the exercise that explains how you can determine pH
|
Test yourself
1. Question: Determine the pH for each of the given solutions.
a. 0.150 M HNO3
b. 0.150 M CH3COOH, Ka = 1.8 × 10-5
c. 0.150 M CHOOH, Ka = 3.5 × 10-4
a. 0.150 M HNO3
b. 0.150 M CH3COOH, Ka = 1.8 × 10-5
c. 0.150 M CHOOH, Ka = 3.5 × 10-4