|
● Most acids have observable characteristic chemical reactions with reactive metals, metal oxides, metal hydroxides, hydrogen carbonates, and carbonates.
Guidance Bases which are not hydroxides, such as ammonia, soluble carbonates, and hydrogen carbonates should be covered. ● Salt and water are produced in exothermic neutralization reactions. |
● Balancing chemical equations for the reaction of acids.
● Identification of the acid and base needed to make different salts. ● Candidates should have experience of acid–base titrations with different indicators. Guidance The colour changes of different indicators are given in the data booklet in section 22. |
|
8.2 How can we deduce balanced acids and base reaction and use them to identify what acids and bases are needed to make a certain salt?
8.2 How can we identify the concentration of an acid or base using titration? |
8.2 What are the observable characteristic chemical reactions of acid with reactive metals, metal oxides, metal hydroxides, hydrogen carbonates, and carbonates?
|
|
There are many acids and bases in a regular household, food, drinks and cleaning supplies to name a few. In this task, you are going to investigate them.
Task: 1. Find at least 2 drinks, 2 foods and 2 cleaning supplies in your house that you think will be acidic or alkaline. Be sure to have at least one item that you think is alkaline. Be as creative as you can, use the images and the video on the right for ideas: 2. Write down balanced equation for at least 4 reaction between any acidic in alkalines items that you have selected in task 1.
3. Describe the reaction that you are thought of in task 2 and share it with your classmate using parlay 4. Read a classmates post on parlay and comment on it giving feedback on: - accuracy of information - Creativity - Clarity of info Student-lab:
Acid and bases are hazardous and can be corrosive. How corrosive are they? Experiment 1: Use the items form task 1 (assuming that all of them are liquids) submerge a small piece of meat in all 6 of them and wait for around 2 days. Make a picture of the pieces of meat afterwards. Experiment 2: Use the items form task 1 (assuming that all of them are liquids) . Measure pieces of eggshell using a kitchen scale. Submerge the eggshells in each of the items and wait for around 2 days. Measure the eggshell pieces again Present and share your raw data in a table on the wakelet |
|
|
Task:
1. Watch the video on how to make your own indicator with red cabbage, as well as how to test substances 2. Make your own indicator. Take a couple of picture of your process 3. Use your indicator to test the pH of household items that you have selected in the student lab above "Acid and bases in your home: student-lab" 4. Take a picture of your results. Please consider the following when you take the picture - Use glass containers (Glasses or glass bowls) so the results are clearly visible - Label each of your household items - Write next to your label of your household item what pH you think it is by looking at the colour of the indicator - try to have all of the items in one picture 5. Upload the your pictures on the wakelet below. |
|
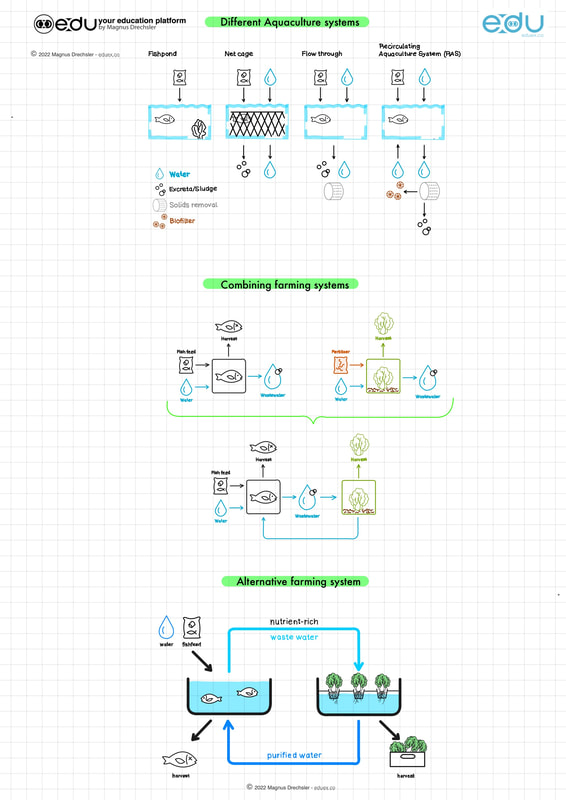
Task:
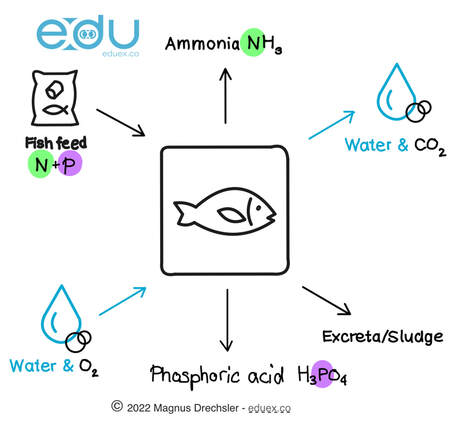
- Study the diagrams below
- Get in groups
- Discuss and research what the diagrams below are illustrating
- Prepare a presentation that explains the diagrams
|
Task:
Effect of ammonia from fish excrements NH3(aq) + H2O(l) -> NH4+(aq) + OH–(aq) Effect of dissociation of phosphoric acid H3PO4(aq) + H2O(l) -> H2PO4-(aq) + H30+(aq) H2PO4-(aq) + H2O(l) -> HPO42-(aq) + H30+(aq) |
|
Task:
|
|