|
● pH = –log[H+(aq)] and [H+] = 10–pH.
● A change of one pH unit represents a 10-fold change in the hydrogen ion concentration [H+]. Guidance Knowing the temperature dependence of Kw is not required. ● pH values distinguish between acidic, neutral, and alkaline solutions. ● The ionic product constant, Kw = [H+][OH–] = 10–14 at 298 K. |
● Solving problems involving pH, [H+], and [OH–].
Guidance ● Students should be concerned only with strong acids and bases in this sub-topic. ● Students will not be assessed on pOH values. ● Students should be familiar with the use of a pH meter and universal indicator. |
Task:
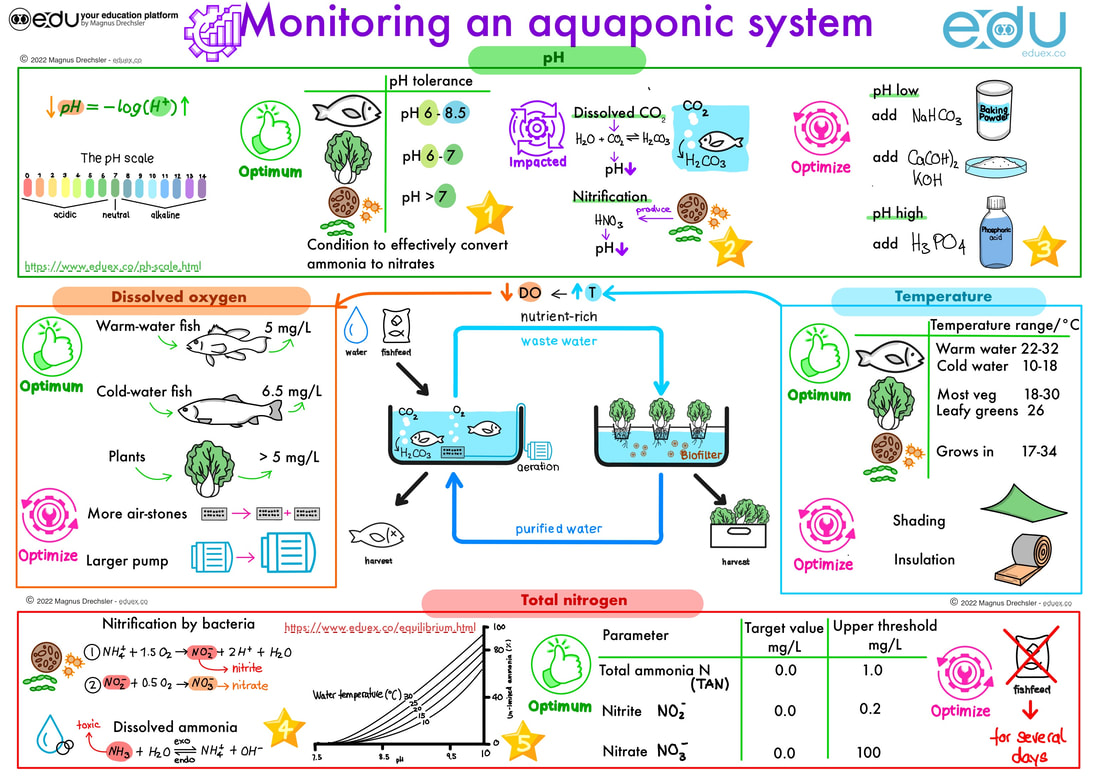
A. Study the image above and explain how to monitor and optimize:
A. Study the image above and explain how to monitor and optimize:
- pH
- Dissolved Oxygen
- Temperature
- Total Nitrogen
- Determine indicators that you can use to test the pH of the water in the aquaculture effectively
- Explain using the pH equation in the image why pH is deceasing when there are more H+ ions in the water.
- Illustrate how the suggested methods in the pH section, optimize the pH by determining neutralisation reactions of these compounds with other acids and alkalis present in an aquaponics system.
- In water there is an equilibrium between the un-ionised ammonia and the ionised ammonium. Explain how this equilibrium is impacted by temperature and pH. Identify how changes to this equilibrium is impacting the fish in the aquaponics system.
- Determine the relationship between un-ionised ammonia, pH and temperature. Use the graph to explain.
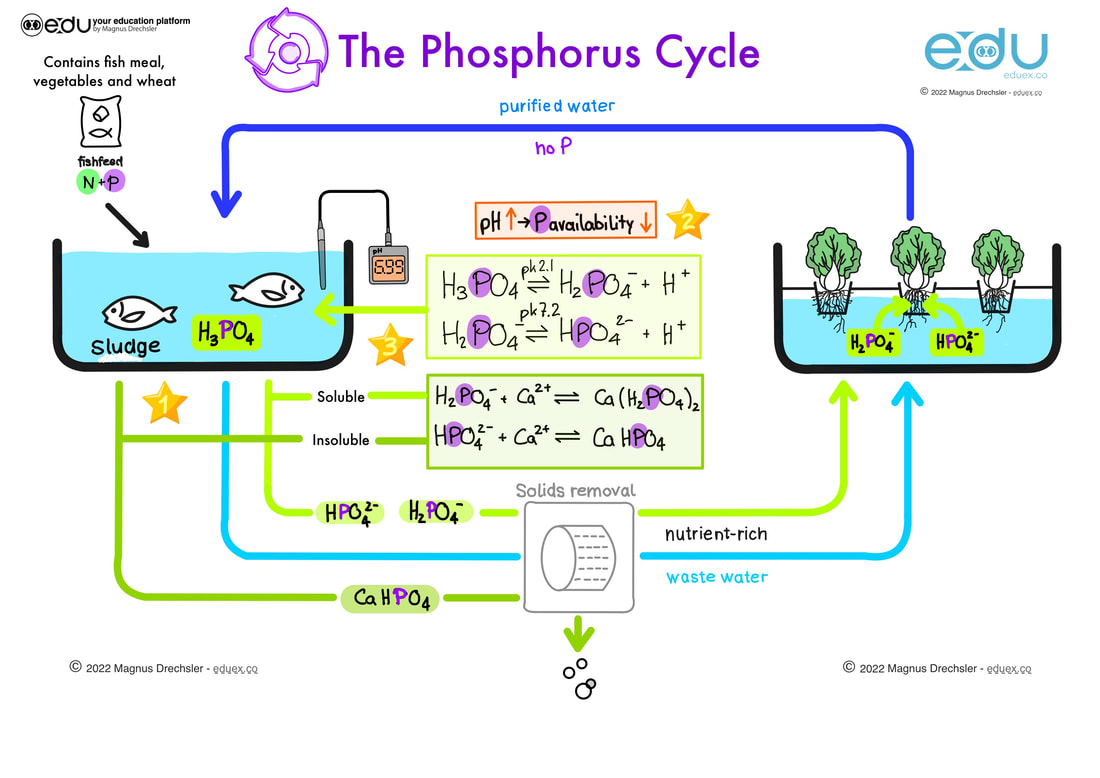
Task:
A. Find the stars with numbers in the image and complete the tasks below:
A. Find the stars with numbers in the image and complete the tasks below:
- SL - Describe how phosphor is circulating in an aquaponics system
- SL - Explain the relationship between pH and P availability by studying the equilibrium reactions in the aquaponics system.
- HL - Explain which hydrogen-phosphate is mainly produced in this aquaponics unit.