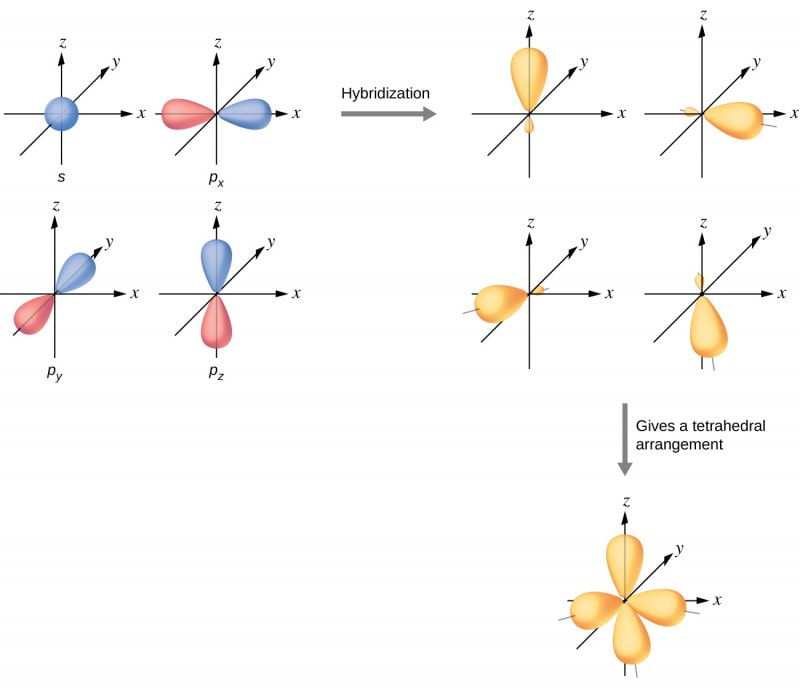
Understandings● A hybrid orbital results from the mixing of different types of atomic orbitals on the same atom.
|
Applications and skills● Explanation of the formation of sp3, sp2, and sp hybrid orbitals in methane, ethene, and ethyne.
● Identification and explanation of the relationships between Lewis (electron dot) structures, electron domains, molecular geometries, and types of hybridization. Guidance Students need only consider species with sp3, sp2, and sp hybridization. |
Hybridization explained - student presentation
|