|
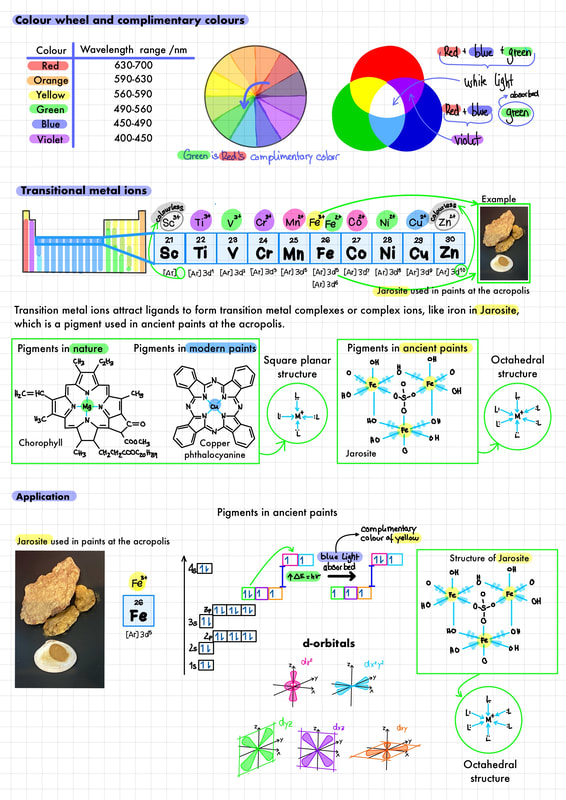
● The d sub-level splits into two sets of orbitals of different energy in a complex ion.
● Complexes of d-block elements are coloured, as light is absorbed when an electron is excited between the d orbitals. ● The colour absorbed is complementary to the colour observed. Guidance The relation between the colour observed and absorbed is illustrated by the colour wheel in the IB Data booklet in section 17. |
● Explanation of the effect of the identity of the metal ion, the oxidation number of the metal, and
the identity of the ligand on the colour of transition metal ion complexes. Guidance Students are not expected to recall the colour of specifi c complex ions. ● Explanation of the effect of different ligands on the splitting of the d orbitals in transition metal complexes and colour observed using the spectrochemical series. Guidance The spectrochemical series is given in the IB data booklet in section 15. A list of polydentate ligands is given in the data booklet in section 16. Students are not expected to know the different splitting patterns and their relation to the coordination number. Only the splitting of the 3-d orbitals in an octahedral crystal fi eld is required. |
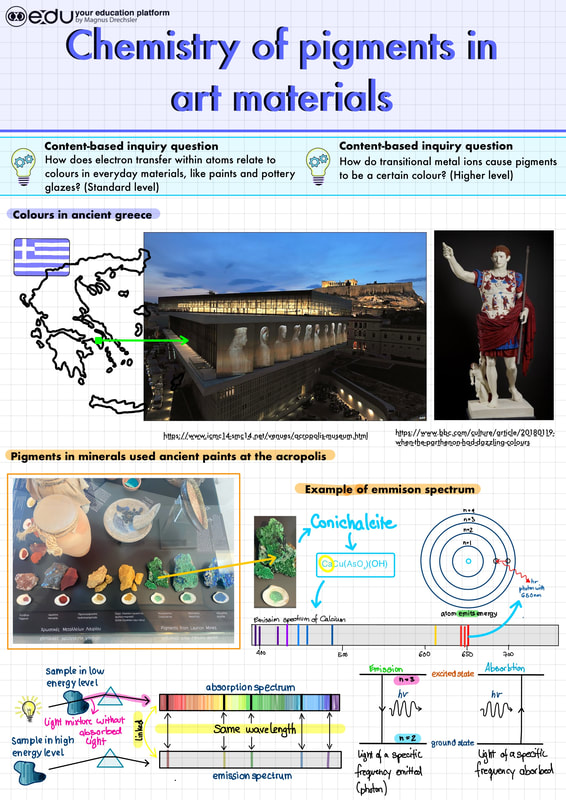
Practical: Colours and pigments in ArtIntroduction to the practical:
This video introduces the colourants that artist are using and explains how views perceive these colorants in Art pieces using the colour wheel. Part 1: Art lab - Pigments and binders:
Task:
Part 2 : Chemistry lab - Testing the absorption of colourants:
Task:
Supporting Research: Experiments on subtractive color mixing with a spectrophotometer
Extra resources:
Collaboration with Visual Arts: Student knowledge/ skills exchangePreparation for student knowledge/skills exchange:
DP Chemistry students:
DP Visual Art students:
| |||||||||||||||
TOKTo what extent does the scientific knowledge of colour impact the viewer's perception of the Art?
To what extent does the scientific knowledge of colour impact the artist in creating Art? |
13.2 Coloured complexes
D-orbital splitting |
|
|
TASK INSTRUCTIONS:
The following factors affect the energy separation between the orbitals and hence the colour of the complex: - Nuclear charge and identity of the central metal - charge density of the ligand - geometry of the complex-ion - number of d electrons and oxidation state of the central metal ion Task 1: Pick one of the factors, research how this factor influences the d orbital splitting, illustrate the information that you have found as visually as possible in poster and presented it in a Flipgrid video. Task 2: Give feedback to other video using the following criteria - How helpful was the visual (Poster) - How clear was the presentation (narration of poster) - How relevant was the information |