|
● Rain is naturally acidic because of dissolved CO2 and has a pH of 5.6. Acid deposition has a pH
below 5.6. ● Acid deposition is formed when nitrogen or sulfur oxides dissolve in water to form HNO3, HNO2, H2SO4, and H2SO3. ● Sources of the oxides of sulfur and nitrogen and the effects of acid deposition should be covered. |
● Balancing the equations that describe the combustion of sulfur and nitrogen to their oxides and the subsequent formation of H2SO3, H2SO4, HNO2, and HNO3.
● Distinction between the pre-combustion and post-combustion methods of reducing sulfur oxide emissions. ● Deduction of acid deposition equations for acid deposition with reactive metals and carbonates. |
Task:
- Get in groups and choose one of the following topics: Causes/ Effects/ Responses to acid deposition
- Research about your topic using any textbook, internet and other sources
- Prepare a presentation to show your research - focus on chemical equations for any chemical process that take place
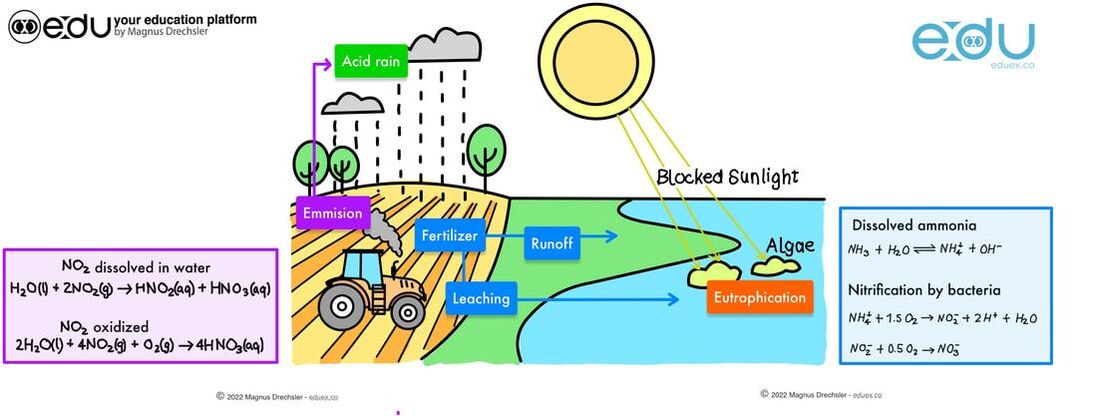
Task:
- Identify issues with tradition farming methods by looking at the image above and using knowledge from this sub-topics as well as other sup-topics from chapter 7 and 8
- Research alternative farming method that address any of the issues
- Present how alternative farming methods address these issues