Understanding● Alkanes: have low reactivity and undergo free radical substitution reactions.
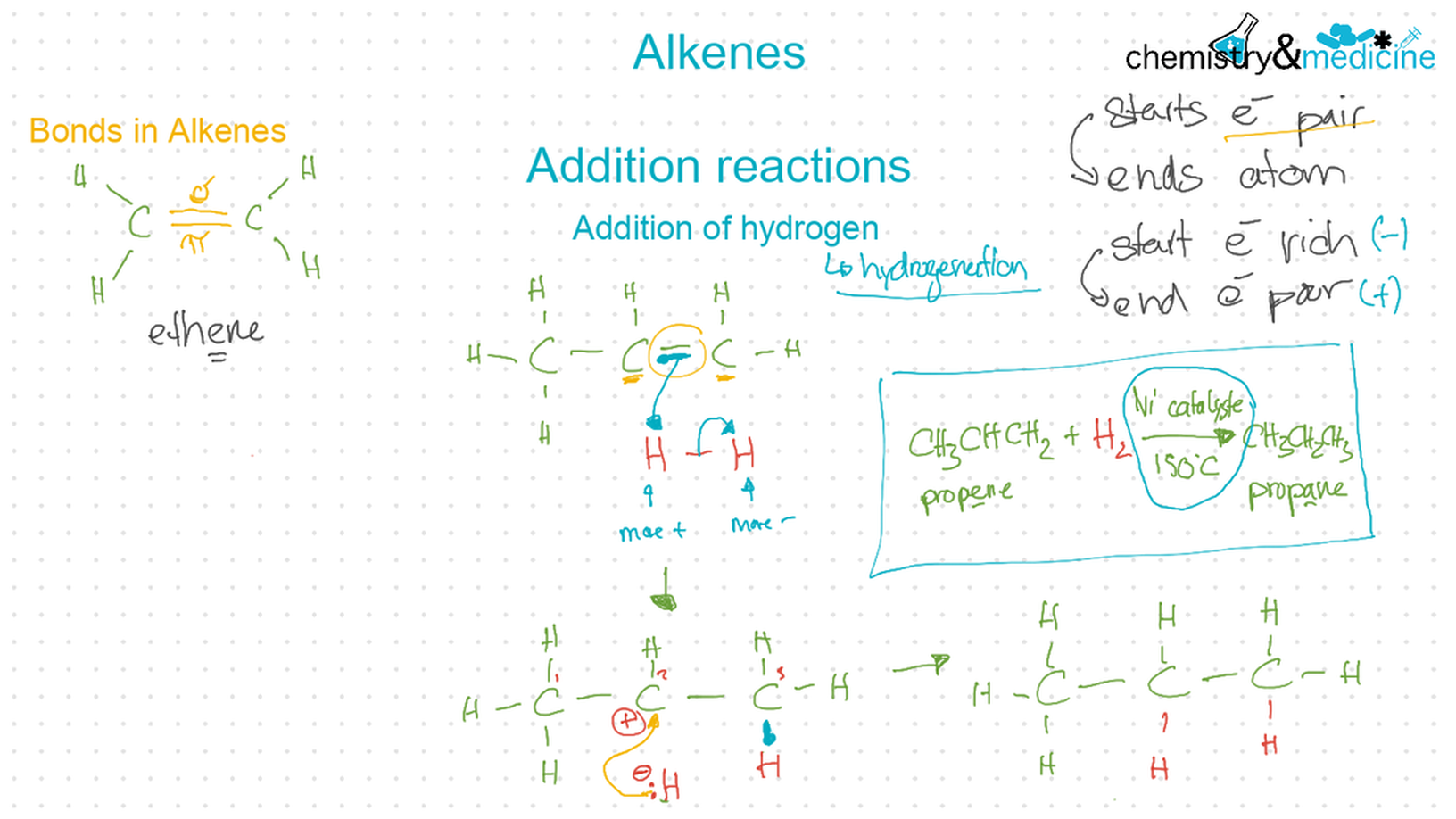
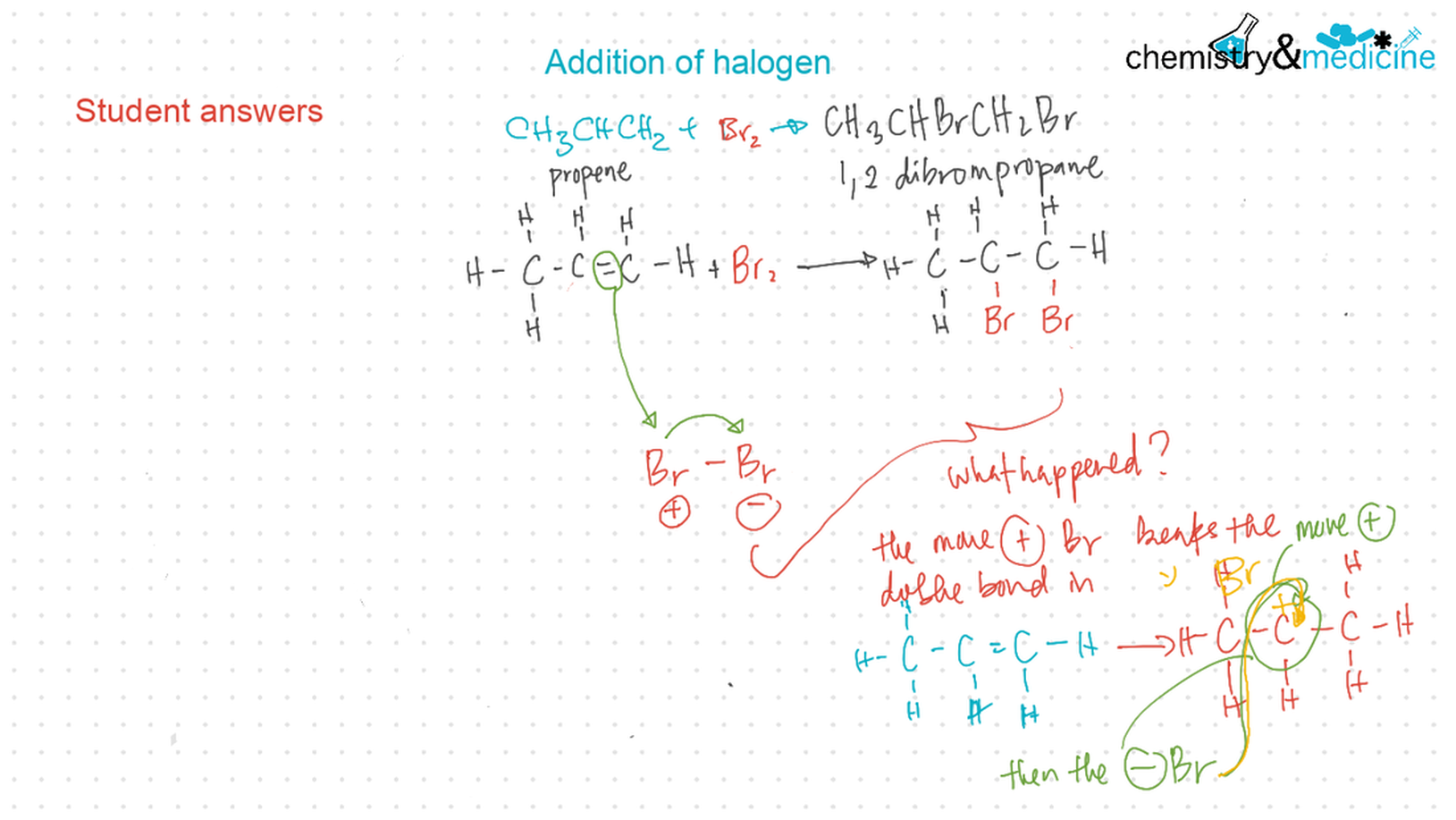
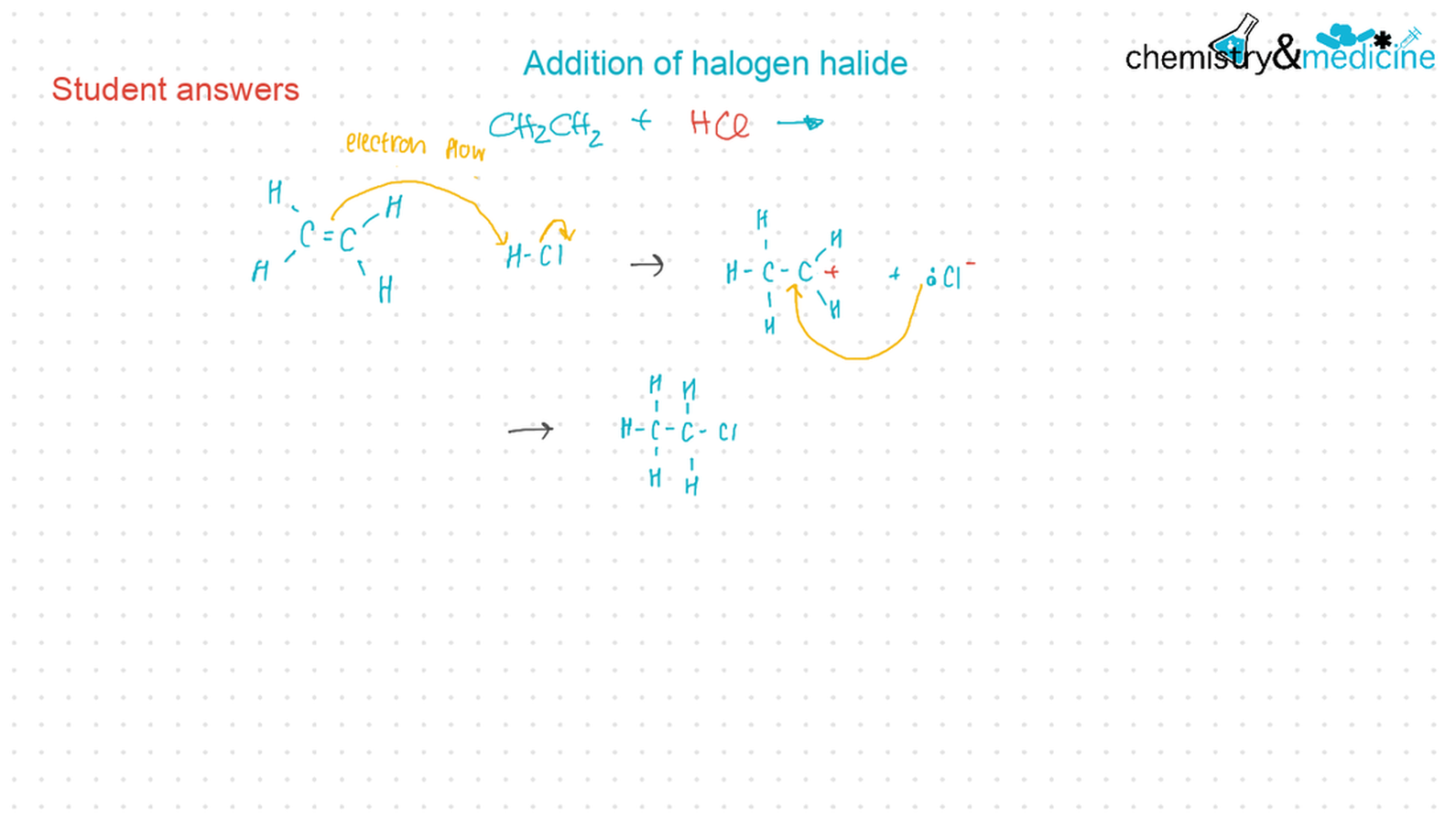
● Alkenes: are more reactive than alkanes and undergo addition reactions. Bromine water can be used to distinguish between alkenes and alkanes. ● Alcohols: undergo nucleophilic substitution reactions with acids (also called esterifi cation or condensation) and some undergo oxidation reactions. ● Halogenoalkanes: are more reactive than alkanes. They can undergo (nucleophilic) substitution reactions. A nucleophile is an electron-rich species containing a lone pair that it donates to an electron-defi cient carbon. ● Polymers: addition polymers consist of a wide range of monomers and form the basis of the plastics industry. ● Benzene: does not readily undergo addition reactions but does undergo electrophilic substitution reactions. |
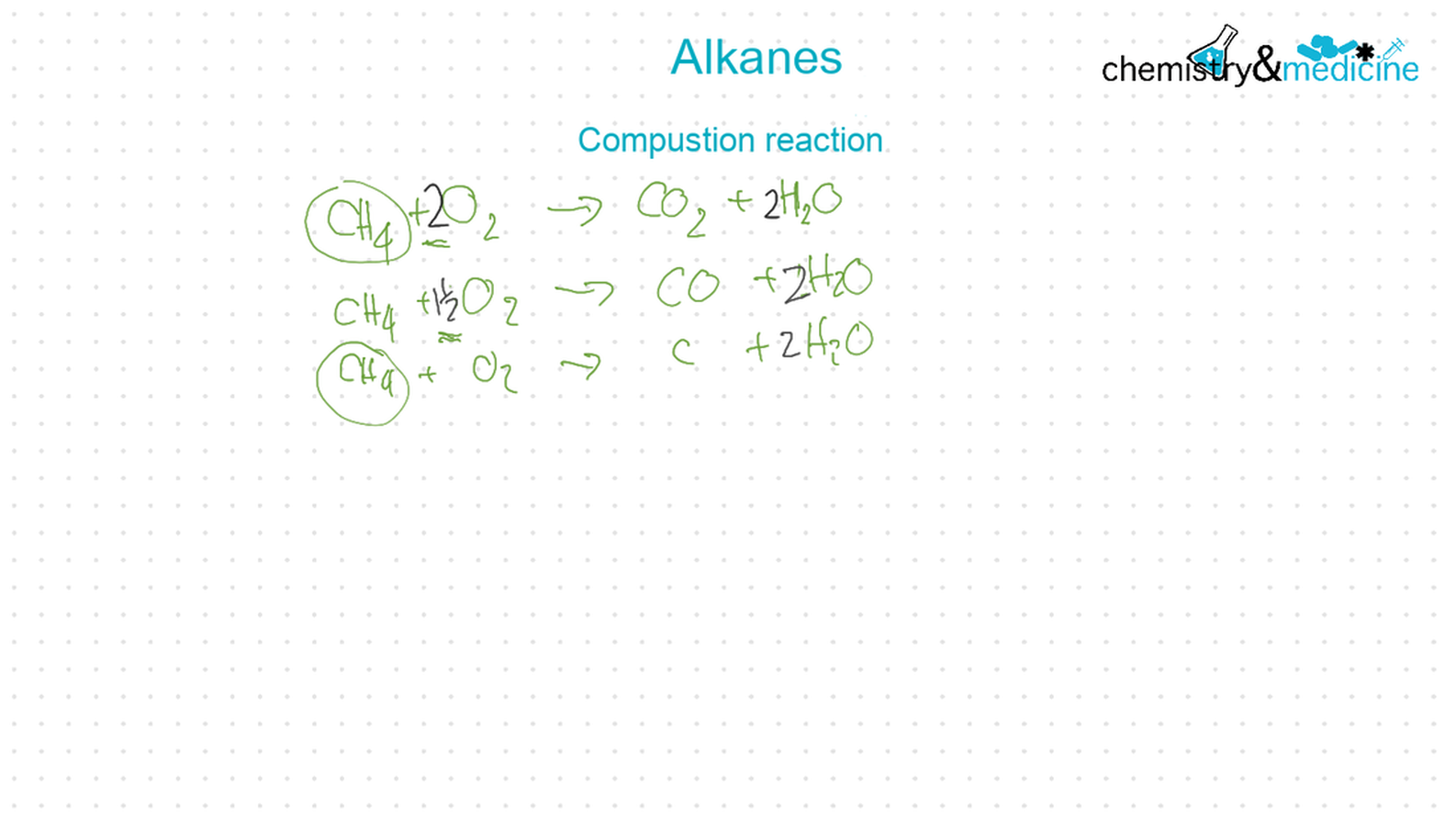
Application and skills● Alkanes:
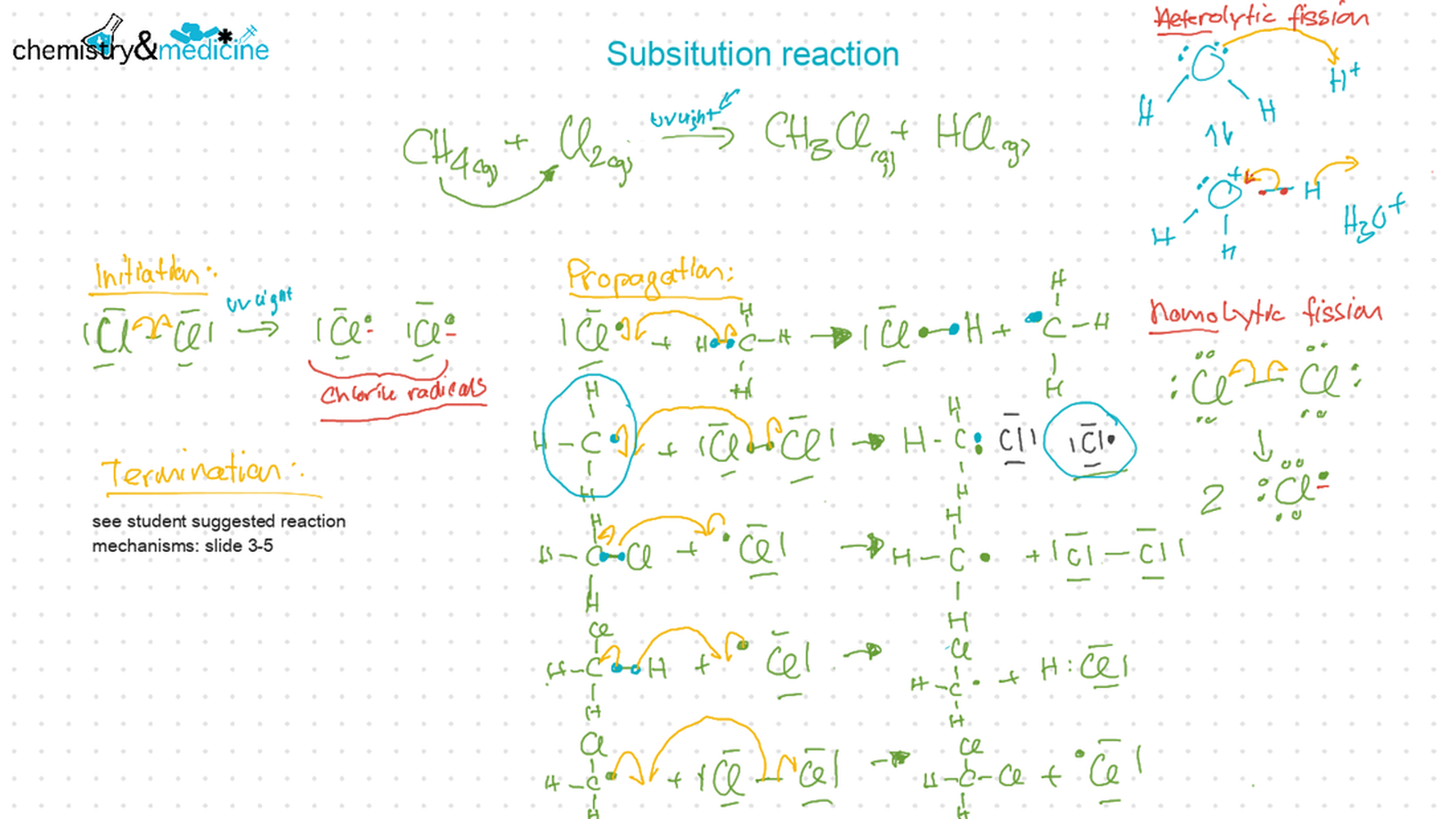
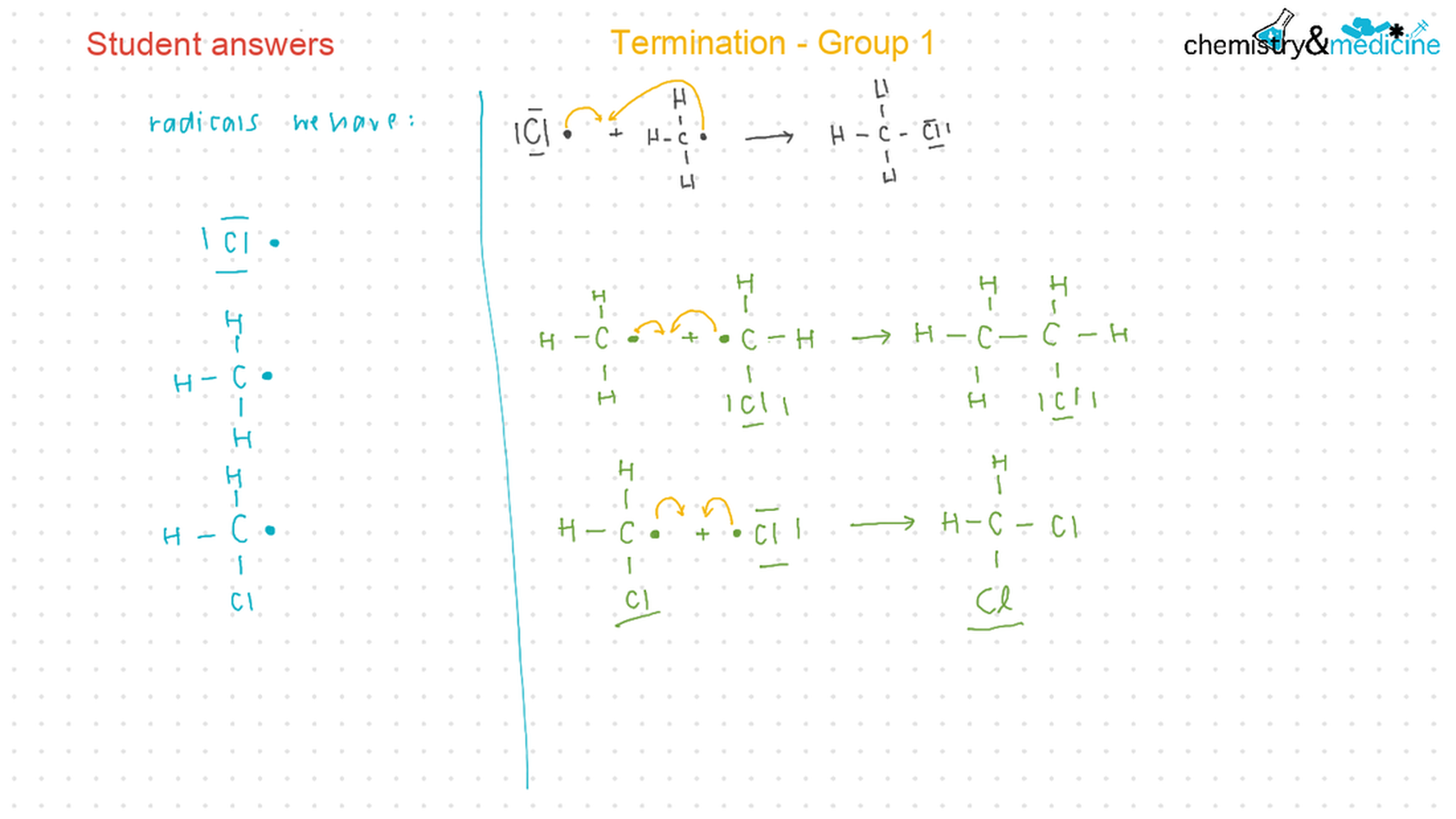
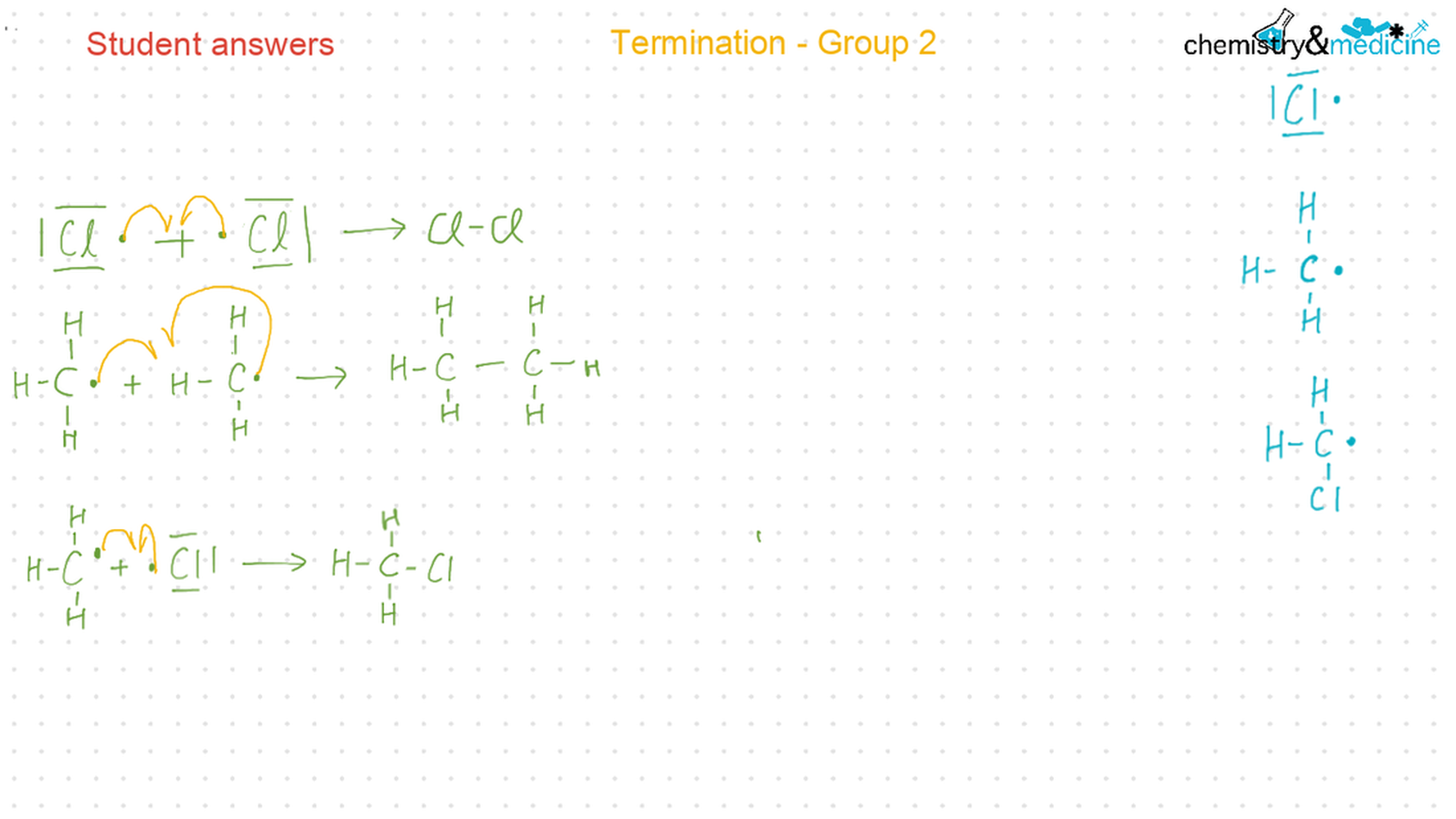
● Writing equations for the complete and incomplete combustion of hydrocarbons. ● Explanation of the reaction of methane and ethane with halogens in terms of a free radical substitution mechanism involving photochemical homolytic fi ssion. ● Alkenes: ● Writing equations for the reactions of alkenes with hydrogen and halogens and of symmetrical alkenes with hydrogen halides and water. ● Outline of the addition polymerization of alkenes. ● Relationship between the structure of the monomer to the polymer and repeating unit. ● Alcohols: ● Writing equations for the complete combustion of alcohols. ● Writing equations for the oxidation reactions of primary and secondary alcohols (using acidifi ed potassium dichromate(VI) or potassium manganate(VII) as oxidizing agents). Explanation of distillation and refl ux in the isolation of the aldehyde and carboxylic acid products. ● Writing the equation for the condensation reaction of an alcohol with a carboxylic acid, in the presence of a catalyst (e.g. concentrated sulfuric acid) to form an ester. ● Halogenoalkanes: ● Writing the equation for the substitution reactions of halogenoalkanes with aqueous sodium hydroxide. Guidance ● Reference should be made to initiation, propagation, and termination steps in free radical substitution reactions. Free radicals should be represented by a single dot. ● The mechanisms of SN1 and SN2 and electrophilic substitution reactions are not required. |
Individual Kahoot recap - functional groupsGame PIN: 01072679
|
Check for understanding: Alkane and alkene class Kahoot |
Alcohols revision
|
Oxidation of alcohols
|