Topic overview in a mindmap
This mind map is being put together by students and will grow with time
1.2 The Mole Concept
Understanding
|
Applications and skills
|
|
Flipped classroom
|
Introducing and calculating relative atomic massThese videos explains what relative atomic mass is and why this mass is relative and what it is relative to. It also recaps on what an isotope is an and how to calculate the relative atomic mass using abundance data of these isotopes.
|
U.2 + AS.1 - Exercise - Relative formula Mass:
|
Syllabus points addressed:
U.2: Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr). AS.1: Calculation of the molar masses of atoms, ions, molecules, formula units. |
P1 - Practical - Food test (Review from MYP - Middle school)This parctical you would probably have done already in middle school. This is just a nice investigation of the food molecules that we will be talking about quite a bit.
|
Chemistry behind the testsPlease note that some of the chemistry is discussed in later units and novel at this point.
Explaining results
|
Avogadro's number
Just like there are 12 apples in a dozen of apples there are 6.02x10^23 atoms in 1 mole. This is important for the next exercise.
U.1 + AS.2 - Exercise - Number of particles to number of moles using avogadro's numbers:
|
Relative formula mass and molar mass
|
|
Converting between Grams and Moles |
Converting between moles, atoms and molecules |
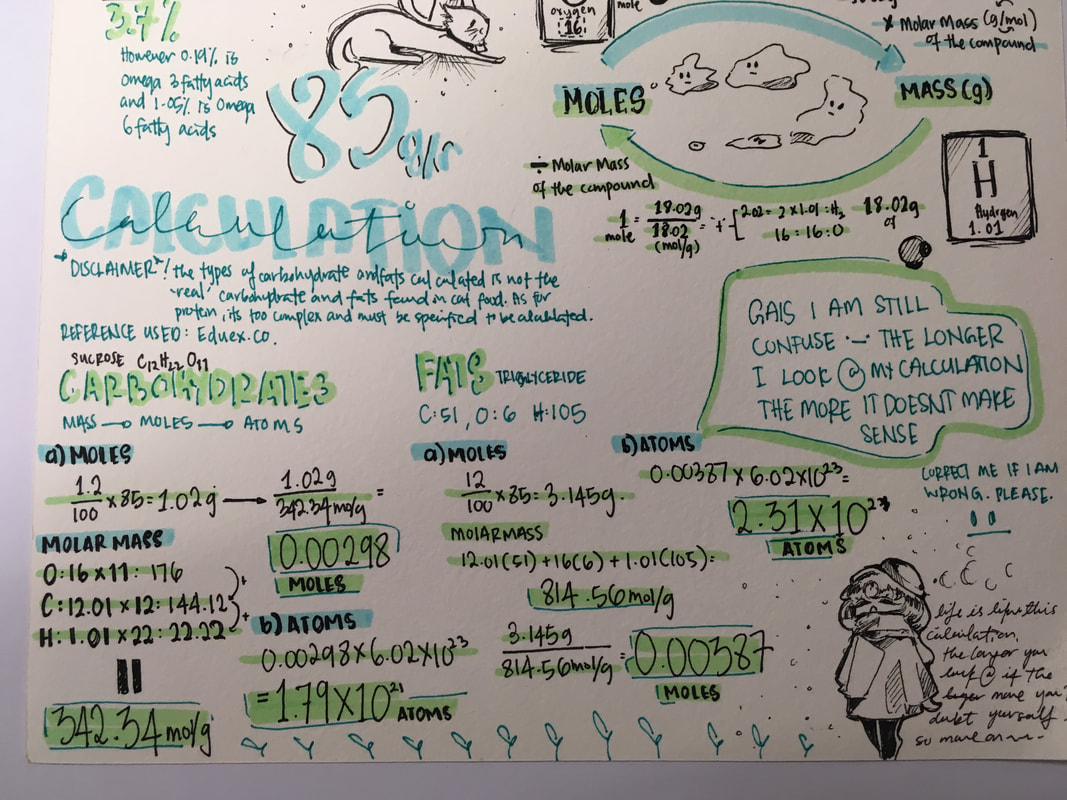
U.3 + AS.2 - Exercise - Mass to number of moles and number of atoms using avogadro's numbers and molar massSee to the right a nutrition label that you would find on your food that you buy from a grocery shop.
|
Syllabus points addressed:
U.3: Molar mass (M) has the units g mol–1. AS.2: Solution of problems involving the relationship between the number of particles, the amount of substance in moles, and the mass in grams. |
|
blended learning
|
U.3 + AS.2 - Nutrition label calculation - student presentation |
Sketchnoting poster example |
Video of student presentation example |
Practice what you have learnedDifferentiation by outcome:
All: Pearson Textbook - Exercise: 13-15 p17 + 16-23 p 21 Most: Oxford Textbook - Quick questions: 1-3 p17-18 Some: Silberberg Textbook - SL Ch 1: 3, 4, 7, 8, 19, 24, 39, 40, 53
|
AS.5 - Determining the empirical formula experimentally (IB Prescribed practical)There are 2 practicals that are determining both the empirical formula of a compound.
Raw data: Follow the instruction, record your data and answer any question on the practical sheet. Fill in your raw data in the form and get your and other students data form the spreadsheet to analyse. Processed data 1. Determine the mean of your and other students data 2. Calculate the mass of magnesium and oxygen 3. Using the practical sheet follow the steps to determine the empirical formula of Magnesium oxide |
Cooking to introduce Percentage composition
|
This is a recipe that we are going to use in our cooking lab later on. This recipe does not use animal fats or proteins. Let's look at the recipe in more detail having percentage composition in mind.
Ingredients
Differentiation by task:
If you are stuck and need help use the videos below and apply the theory to this real life problem. |
AS.3 - Percentage composition
Videos explain how to determine the empirical formula using the percentage composition.
|
|
|
AS.3 - Percentage composition
|
|
Flipped classroom
|
U.4 + AS.4 - Empirical and molecular FormulaThe video explains the difference between the molecular and empirical formula. It demonstrates how to derive the empirical formula from the molecular formula, giving a number of examples.
|
U.4 + AS.4 - Determining the empirical and molecular formula
|