16.1 Rate expression and reaction mechanism
|
|
Additional resources
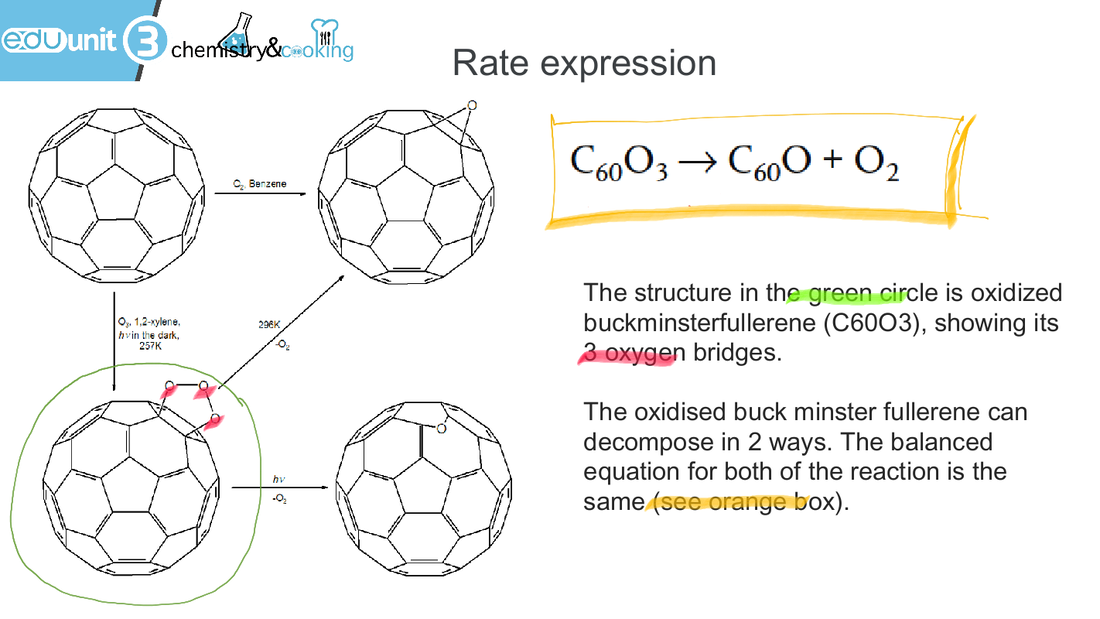
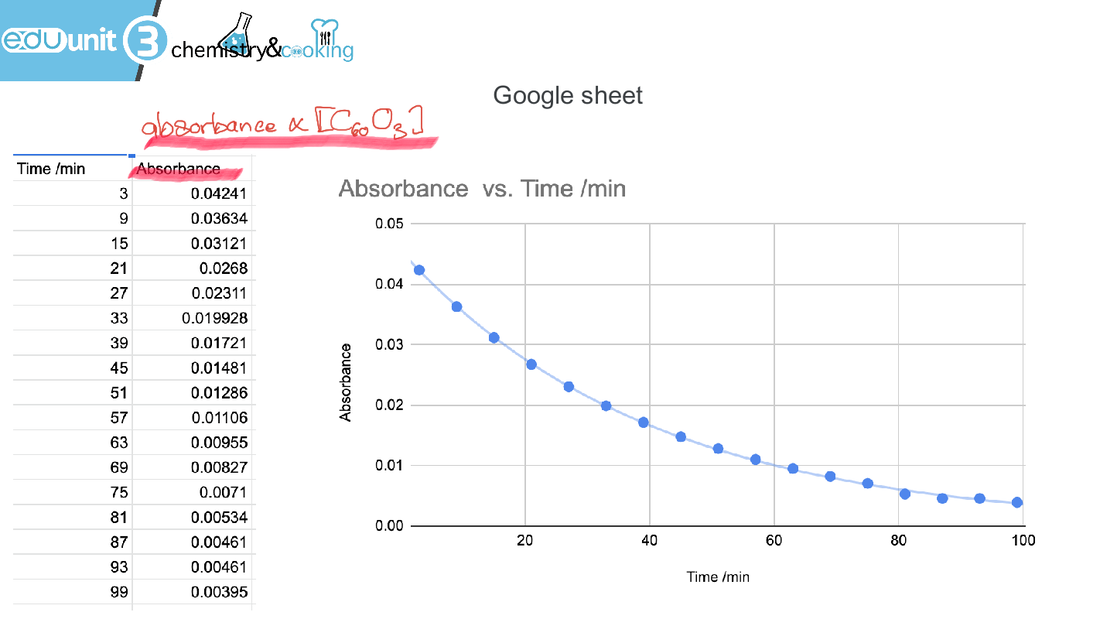
See below the experimental data of the reaction. We measured the absorbance of light of a certain wavelength over time. Please note that the absorbance of light is directly proportional to the concentration of the oxidised buckminsterfullerene.
Task:
- Access the google spreadsheet and make your own copy - use the link below
- Use the spreadsheet functions to calculate Δ t and Δ [C60O3] - for help look at the model tab in the spreadsheet
- Use the spreadsheet functions to calculate rate (rate = -(Δ [C60O3]/Δ t) - for help look at the model tab in the spreadsheet
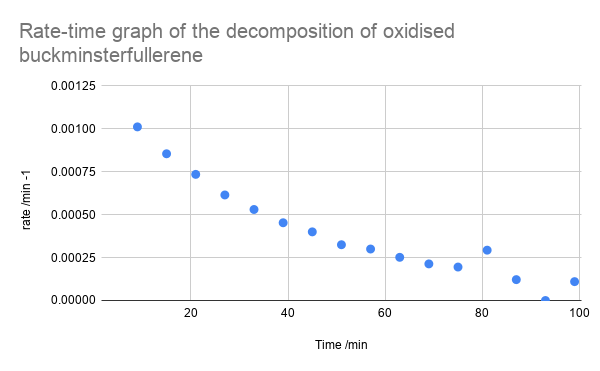
- Plot a rate-time graph - see below what it should look like
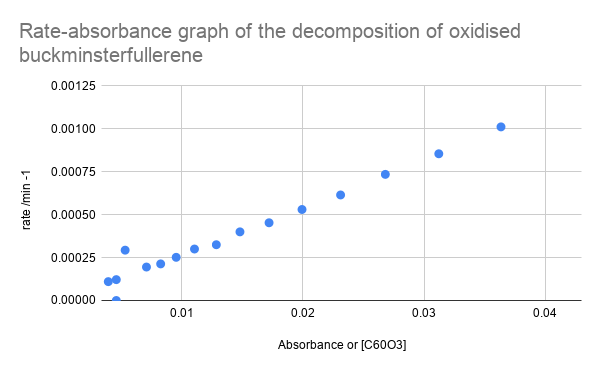
- Plot a rate-absorbance graph of the decomposition of the oxidised buckminsterfullerene - see below what it should look like
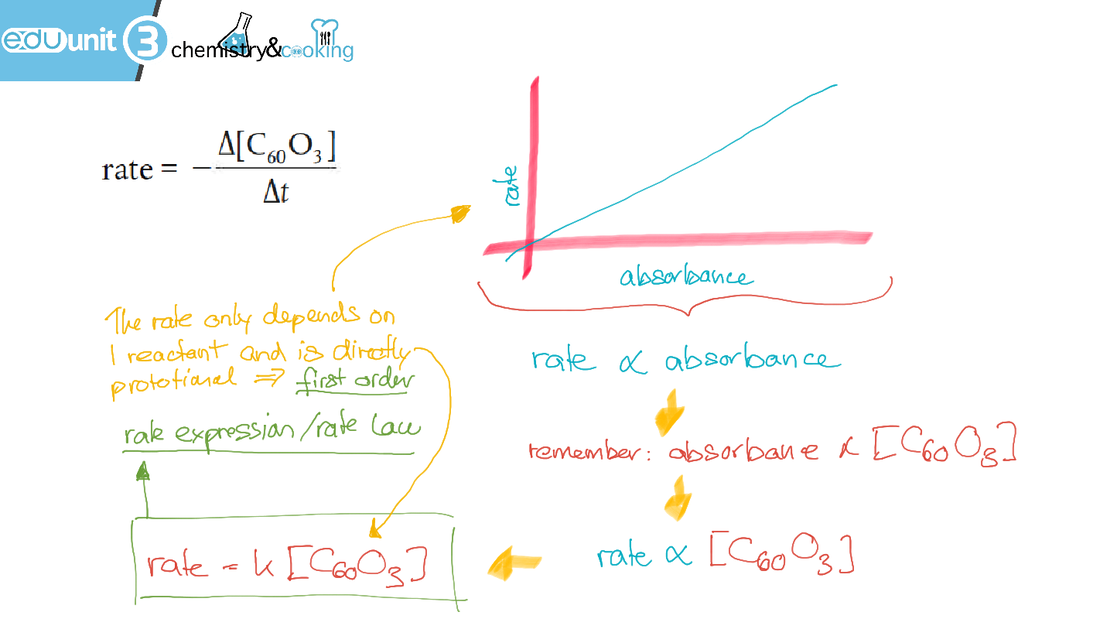
The rate-absorbance graph demonstrates that the the rate is proportional to absorbance, which means that rate is proportional to the concentration of oxidised buckminsterfullerene. This will help us to determine the rate expression of the raction, see below:
Task:
1. Watch the video
2. Use the data in the poster and create a table in a spreadsheet
3. Using the volume of CO2 calculate the ∆ [NaHCO3]
4. Plot the ∆ [NaHCO3] over time
5. Determine the rate of reaction by drawing a tangent at at least 6 points in time
6. Use your data to plot the following
- a) Rate over time
- b) Rate vs Concentration
7. Determine the order of reaction
8. Explain your working using the flipgrid below
9. Give feedback to your peers
1. Watch the video
2. Use the data in the poster and create a table in a spreadsheet
3. Using the volume of CO2 calculate the ∆ [NaHCO3]
4. Plot the ∆ [NaHCO3] over time
5. Determine the rate of reaction by drawing a tangent at at least 6 points in time
6. Use your data to plot the following
- a) Rate over time
- b) Rate vs Concentration
7. Determine the order of reaction
8. Explain your working using the flipgrid below
9. Give feedback to your peers
More information about determining rates, reaction mechanism & rae expressionTask:
1. Form and pick on of the topics below - Determination of the order of reaction (pearson textbook page 294-5) - Reaction mechanism (pearson textbook page 296-8) - Rate expression (pearson textbook page 298-9) 2. Research your topic using the textbook, internet and other sources 3. Create a presentation with the following items - diagram and tables to support understanding - worked example - one questions for students to answer about your topic |