|
Discuss why the different metal salts burn in different colours by using the theory of quantisation of energy and the information in the video "Energy levels - AS Quantum". Read your classmates comments and comment yourself on the blog post "Flame test - Application". Ensure that you do not repeat what has been said already.
8 Comments
Question: Energy levels and emission spectrum
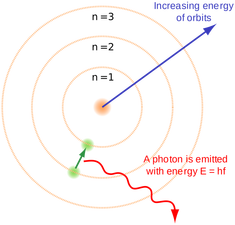
Look at the picture of the hydrogen line emission spectrum (figure 1) below and discuss why this emission spectra has only certain lines rather than the whole spectra with all the colours. Use the video "Energy levels - AS Quantum" and figure 2 to help you explain using scientific reasoning. Read your classmates comments and comment yourself. Ensure that you do not repeat what has been said already.
|
ArchivesCategories |
- Home
-
DP Chemistry
-
DP course material
>
- chemistry&sustainability >
-
Chemistry & materials
>
- nuclear atom
- Electron configuration
- Electrons in atoms
- Periodic table
- Periodic trends
- D-Block elements
- Coloured complexes
- Exploration - Bonding
- Ionic bonding and structure
- Covalent bonding
- Covalent structure
- Intermolecular forces
- Metallic bonding
- Further covalent bonding
- Hybridization
- Transfer goal - materials
- Chemistry&materials-escape room
- chemistry&cooking >
- chemistry&industry >
-
chemistry&medicine
>
- DP online resources
-
DP course material
>
- MYP Science
- Sustainability
- Educators lounge
- membership
- Home
-
DP Chemistry
-
DP course material
>
- chemistry&sustainability >
-
Chemistry & materials
>
- nuclear atom
- Electron configuration
- Electrons in atoms
- Periodic table
- Periodic trends
- D-Block elements
- Coloured complexes
- Exploration - Bonding
- Ionic bonding and structure
- Covalent bonding
- Covalent structure
- Intermolecular forces
- Metallic bonding
- Further covalent bonding
- Hybridization
- Transfer goal - materials
- Chemistry&materials-escape room
- chemistry&cooking >
- chemistry&industry >
-
chemistry&medicine
>
- DP online resources
-
DP course material
>
- MYP Science
- Sustainability
- Educators lounge
- membership




 RSS Feed
RSS Feed
